Publication
834
Faraday Discuss., 203, 301-313, 2017
DOI:10.1039/C7FD00082K
|
|
|
|
|
|
|
Comparative study of non-covalent interactions between cationic N-phenylviologens and halides by electrochemistry and NMR: the halogen bonding effect
|
|
|
|
Geordie Creste, Sihem Groni, Claire Fave, Mathieu Branca, and Bernd Schöllhorn
Laboratoire d’Electrochimie Moléculaire, UMR CNRS 7591, Université Paris Diderot, Sorbonne Paris Cité, Bâtiment Lavoisier, 15 rue Jean-Antoine de Baïf, 75205 Paris Cedex 13, France
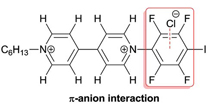
Five N-phenylviologen (PV2+) derivatives have been synthesized and their electrochemical behavior in the presence of halide anions has been studied. Further investigations were carried out by 1H and 19F NMR spectroscopy at different chloride concentrations. This is the first time a systematic study combines cyclic voltammetry and NMR spectroscopy in order to analyse the contribution of halogen bonding among the various non-covalent interactions between iodinated N-phenylviologens. The results show strong evidence for a significant “halogen bonding effect” in the interaction between halides and the iodo-tetrafluoro-phenylviologen PV2+-C6F4I. A significant influence of halogen bonding on reduction potentials of the novel halogen bond donor PV2+-C6F4I has been evidenced resulting in the first example of “inverse redox switching” of an XB-donor being partially deactivated upon reduction. Furthermore the particular binding properties of the perfluorinated derivative PV2+-C6F5 towards chloride are discussed considering a possible contribution of π–anion interaction in solution. |

