Publication
835
Nat. Mater., 16 ,1016--1021, 2017
DOI:10.1038/nmat4968
|
|
|
|
|
|
|
Nanodiffusion in electrocatalytic films
|
|
|
|
Cyrille Costentin, Carlo Di Giovanni, Marion Giraud, Jean-Michel Savéant, and Cédric Tard
Université Paris Diderot, Sorbonne Paris Cité, Laboratoire d’Electrochimie Moléculaire, Unité Mixte de Recherche Université—CNRS No 7591, Bâtiment Lavoisier, 15 rue Jean de Baïf, 75205 Paris Cedex 13, France
Université Paris Diderot, Sorbonne Paris Cité, Laboratoire ITODYS, Unité Mixte de Recherche Université—CNRS No 7086, Bâtiment Lavoisier, 15 rue Jean de Baïf, 75205 Paris Cedex 13, France
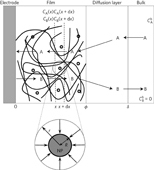
In the active interest aroused by electrochemical reactions’ catalysis, related to modern energy challenges, films deposited on electrodes are often preferred to homogeneous catalysts. A particularly promising variety of such films, in terms of efficiency and selectivity, is offered by sprinkling catalytic nanoparticles onto a conductive network. Coupled with the catalytic reaction, the competitive occurrence of various modes of substrate diffusion—diffusion toward nanoparticles (‘nanodiffusion’) against film linear diffusion and solution linear diffusion—is analysed theoretically. It is governed by a dimensionless parameter that contains all the experimental factors, thus allowing one to single out the conditions in which nanodiffusion is the dominant mode of mass transport. These theoretical predictions are illustrated experimentally by proton reduction on a mixture of platinum nanoparticles and carbon dispersed in a Nafion film deposited on a glassy carbon electrode. The density of nanoparticles and the scan rate are used as experimental variables to test the theory. |

