Publication
838
ChemSusChem, 10 (20), 4009-4013, 2017
DOI:10.1002/cssc.201701354
|
|
|
|
|
|
|
Photocatalytic Conversion of CO2 to CO by a Copper(II) Quaterpyridine Complex
|
|
|
|
Zhenguo Guo, Fei Yu, Ying Yang, Chi-Fai Leung, Siu-Mui Ng, Chi-Chiu Ko, Claudio Cometto, Tai-Chu Lau, and Marc Robert
Department of Science and Environmental Studies, The Education University of Hong Kong, Hong Kong, P. R. China
nstitute of Molecular Functional Materials and Department of Chemistry, City University of Hong Kong, Kowloon Tong, Hong Kong, P. R. China
Université Paris Diderot, Sorbonne Paris Cité, Laboratoire Electrochimie Moléculaire, Unité Mixte de Recherche Université-CNRS no. 7591, Paris Cedex 13, France
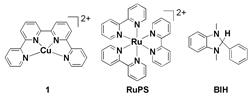
The invention of efficient systems for the photocatalytic reduction of CO2 comprising earth-abundant metal catalysts is a promising approach for the production of solar fuels. One bottleneck is to design highly selective and robust molecular complexes that are able to transform the CO2 gas. The CuII quaterpyridine complex [Cu(qpy)]2+ (1) is found to be a highly efficient and selective catalyst for visible-light driven CO2 reduction in CH3CN using [Ru(bpy)3]2+ (bpy: bipyridine) as photosensitizer and BIH/TEOA (1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole/triethanolamine) as sacrificial reductant. The photocatalytic reaction is greatly enhanced by the presence of H2O (1–4 % v/v), and a turnover number of >12 400 for CO production can be achieved with 97 % selectivity, which is among the highest of molecular 3d CO2 reduction catalysts. Results from Hg poisoning and dynamic light scattering experiments suggest that this photocatalyst is homogenous. To the best of our knowledge, 1 is the first example of molecular Cu-based catalyst for the photoreduction of CO2. |

