Publication
839
J. Phys. Chem. C, 121 (41) ,23207-23217, 2017
DOI:10.1021/acs.jpcc.7b07071
|
|
|
|
|
|
|
Investigating Charge Transfer in Functionalized Mesoporous EISA–SnO2 Films
|
|
|
|
Wael Hamd, Christel Laberty-Robert, François Lambert, Cyrille Costentin, Benoît Limoges, and Véronique Balland
Laboratoire d’Electrochimie Moléculaire, UMR 7591 CNRS, Université Paris Diderot, Sorbonne Paris Cité, 15 rue Jean-Antoine de Baïf, Paris F-75205 Cedex 13, France
Sorbonne Universités, UPMC Univ Paris 6, CNRS, Collège de France, Laboratoire de Chimie de la Matière Condensée de Paris, 4 place Jussieu, Paris F-75005, France
Laboratoire des Biomolécules, UMR 7203 CNRS, UPMC Univ Paris 6, Ecole Normale Supérieure, 24 rue Lhomond, Paris F-75005, France
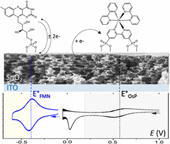
Semiconductive transparent thin films of periodically organized nanostructured SnO2 were prepared on flat conductive ITO substrates by evaporation-induced self-assembly (EISA) under different dip-coating regimes and then functionalized by two redox-active chromophores, i.e., the flavin mononucleotide (FMN) able to reversibly exchange 2 e– and 2 H+ and the [OsII(bpy)2(4,4'-CH2PO3H2-bpy)]2+ complex (OsP) involving a fast and reversible one-electron transfer. The redox behavior of these two chemisorbed chromophores was investigated by cyclic voltammetry and cyclic voltabsorptometry. On account of the distinct formal potential of the two redox chromophores relative to the position of the lower conduction band edge of SnO2, the heterogeneous electron transfer was observed to be either reversible (FMN) or irreversible (OsP). In the case of the OsP-functionalized SnO2 electrode, quantitative analysis of the cyclic voltabsorptograms was achieved within the framework of our previously proposed kinetic model of charge transfer/transport in mesoporous semiconductive films, allowing for direct comparison between EISA–TiO2 and EISA–SnO2 electrodes. It is notably shown that the interfacial electron transfer between the adsorbed redox chromophore and the SnO2 interface is the rate-determining process under our experimental conditions. It is additionally demonstrated that the electrons trapped in the low-energy surface states of EISA–SnO2 can directly participate in the interfacial electron transfer, a behavior that strongly contrasts that which we had previously found at EISA–TiO2 electrodes (i.e., wherein only electrons from the conduction band were involved in the interfacial electron transfer). |

