Publication
843
Nat. Rev. Chem., 1, 0087, 2017
DOI:10.1038/s41570-017-0087
|
|
|
|
|
|
|
Towards an intelligent design of molecular electrocatalysts
|
|
|
|
Cyrille Costentin and Jean-Michel Savéant
Université Paris Diderot, Sorbonne Paris Cité, Laboratoire d’Electrochimie Moléculaire, Unité Mixte de Recherche Université-CNRS No. 7591, Bâtiment Lavoisier, 15 Rue Jean de Baïf, 75205 Paris Cedex 13, France
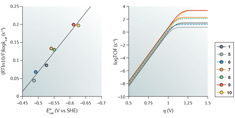
There is a need for the quantitative assessment of molecular electrocatalysts, particularly those designed to address modern energy challenges. This calls for systematic benchmarking to help rationalize the wealth of available data and to apply the lessons learnt to the innovative design of more efficient catalysts. For these purposes, it is tempting to examine the relationship between the reaction kinetics and the stabilization of a single primary intermediate by constructing a volcano plot. The attractiveness and inherent flaw in this oversimplified approach stem from accounting for complicated multi-electron, multistep processes with a single descriptor. In this Perspective, we instead advocate correlating the turnover frequency with the overpotential through a catalytic Tafel plot, which provides a much less restrictive and more reliable evaluation of intrinsic catalyst performance. Within this framework, one way to optimize molecular catalysts involves altering ligand substituents to tune the electronic structure of the catalyst. These inductive through-structure effects are subject to an ‘iron law’, which dictates that gains in the form of smaller overpotentials come at the price of reduced turnover frequencies (and vice versa). This law can be circumvented by instead engineering through-space effects that stabilize the primary catalytic intermediate. These considerations should be based on the patient gathering of reliable experimental data obtained upon variation of the maximal number of operational parameters. |

