Publication
848
ACS Energy Lett., 3, 695-703, 2018
DOI:10.1021/acsenergylett.8b00008
|
|
|
|
|
|
|
Catalysis of CO2 Electrochemical Reduction by Protonated Pyridine and Similar Molecules. Useful Lessons from a Methodological Misadventure
|
|
|
|
Cyrille Costentin, Jean-Michel Savéant, and Cédric Tard
Université Paris Diderot, Sorbonne Paris Cité, Laboratoire d’Electrochimie Moléculaire, Unité Mixte de Recherche Université - CNRS N° 7591, Bâtiment Lavoisier, 15 rue Jean de Baïf, 75205 Paris Cedex 13, France
LCM, CNRS, Ecole Polytechnique, Université Paris-Saclay, 91128 Palaiseau Cedex, France
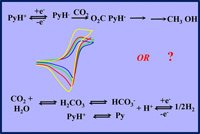
Electrochemical reduction of carbon dioxide is a particularly important issue in energy and environment contemporary challenges. It mobilized for several decades many researchers in the fields of catalysis by low-valent transition metal complexes. In this context, the report that a molecule as simple as protonated pyridine could catalyze the 6e– + 6H+ conversion of CO2 into methanol and triggered quite a lot of interest and amazement, even if the Faradaic yield was relatively modest (20%) and a precious metal (Pt) had to be used as an electrode material. Successive investigations of the same system or of systems involving molecules similar to protonated pyridine produced divergent results. The amount of data available at present from several research groups is sufficiently large to try to take stock of the situation. This concerns first and foremost a critical examination of the nature and Faradaic yields of electrolysis products. It also deals with the extensive use in this research of a nondestructive electrochemical technique – cyclic voltammetry – as a footprint of the existence of the catalysis process and a way to access its mechanism. As shown here, serious methodological problems arise in this connection, whose discussion may serve as general guidelines for safer future use of cyclic voltammetry. The same type of methodological questions also result from the theoretical computations that have abundantly accompanied experimental reports. |

