Publication
852
J. Inorg. Biochem., 8, 10–16, 2018
DOI:10.1016/j.jinorgbio.2018.04.023
|
|
|
|
|
|
|
Functional modeling of the MnCAT active site with a dimanganese(III) complex of an unsymmetrical polydentate N3O3 ligands
|
|
|
|
Gabriela N. Ledesma, Elodie Anxolabéhère–Mallart, Laurent Sabater, Christelle Hureau, and Sandra R. Signorella
IQUIR (Instituto de Química Rosario), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Facultad de Ciencias Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario, Suipacha 531, S2002LRK Rosario, Argentina
Laboratoire d'Electrochimie Moléculaire UMR CNRS–P7 7591, Université Paris Diderot–Paris, 15 rue Jean–Antoine de Baïf, 75205 Paris Cedex 13, France
CNRS, LCC (Laboratoire de Chimie de Coordination) and UPS, INPT, LCC, Université de Toulouse, 205 route de Narbonne, F–31077 Toulouse, France
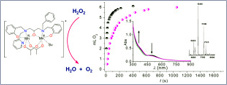
A new diMnIII complex, [Mn2L(OAc)2(H2O)](BPh4)·3H2O (1), obtained with the unsymmetrical N3O3-ligand H3L = 1-[N-(2-pyridylmethyl),N-(2-hydroxybenzyl)amino]-3-[N′-(2-hydroxybenzyl),N′-(benzyl)amino]propan-2-ol, has been prepared and characterized. The unsymmetrical hexadentate ligand L3− leads to coordination dissymmetry (dissimilar donor atoms) around each Mn ion (N2O4 and NO4(solvent), respectively) leaving one labile site on one of the two Mn ions that facilitates interaction of the metal center with H2O2, as in Mn catalase. 1 is able to catalyze H2O2 disproportionation in acetonitrile, with second-order rate constant kcat = 23.9(2) M−1 s−1. The accessibility of the MnII2 state and the closeness of the two one-electron reduction processes suggest 1 employs MnIII2/MnII2 oxidation states for catalysis . |

