Publication
855
Biochemistry, 57 (24), 13402-13415, 2018
DOI:10.1021/acs.biochem.8b00160
|
|
|
|
|
|
|
Properties of Site-Specifically Incorporated 3-Aminotyrosine in Proteins To Study Redox-Active Tyrosines: Escherichia coli Ribonucleotide Reductase as a Paradigm
|
|
|
|
Wankyu Lee, Müge Kasanmascheff, Michael Huynh, Anthony Quartararo, Cyrille Costentin, Isabel Bejenke, Daniel G. Nocera, Marina Bennati, Cecilia Tommos, and JoAnne Stubbe
Department of Chemistry and ‡Department of Biology, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, United States
Max Planck Institute for Biophysical Chemistry, Am Fassberg 11, Göttingen, 37077 Germany
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, Massachusetts 02138 United States
Laboratoire d’Electrochimie Moléculaire, Unité Mixte de Recherche Université − CNRS No 7591, Université Paris Diderot, Sorbonne Paris Cité, Bâtiment Lavoisier, 15 rue Jean de Baïf, 75205 Paris Cedex 13, France
Department of Biochemistry and Biophysics, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania 19104, United States
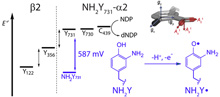
3-Aminotyrosine (NH2Y) has been a useful probe to study the role of redox active tyrosines in enzymes. This report describes properties of NH2Y of key importance for its application in mechanistic studies. By combining the tRNA/NH2Y-RS suppression technology with a model protein tailored for amino acid redox studies (alpha3X, X = NH2Y), the formal reduction potential of NH2Y32(O•/OH) (E°´ = 395 ± 7 mV at pH 7.08 ± 0.05) could be determined using protein film voltammetry. We find that the differenceE°´between NH2Y32(O•/OH) and Y32(O•/OH) when measured under reversible conditions is ¨300–400 mV larger than earlier estimates based on irreversible voltammograms obtained on aqueous NH2Y and Y. We have also generated D6-NH2Y731-alpha2 of ribonucleotide reductase (RNR), which when incubated with beta2/CDP/ATP generates the D6-NH2Y731•-alpha2/beta2 complex. By multifrequency electron paramagnetic resonance (35, 94, and 263 GHz) and 34 GHz 1H ENDOR spectroscopies, we determined the hyperfine coupling (hfc) constants of the amino protons that establish RNH2• planarity and thus minimal perturbation of the reduction potential by the protein environment. The amount of Y in the isolated NH2Y-RNR incorporated by infidelity of the tRNA/NH2Y-RS pair was determined by a generally useful LC-MS method. This information is essential to the utility of this NH2Y probe to study any protein of interest and is employed to address our previously reported activity associated with NH2Y-substituted RNRs. |

