Publication
860
Dalton Trans., 47 (34), 11903-11908, 2018
DOI:10.1039/C8DT01598H
|
|
|
|
|
|
|
Oxygen activation at a dicobalt centre of a dipyridylethane naphthyridine complex
|
|
|
|
Casey N. Brodsky, Guillaume Passard, Andrew M. Ullman, David E. Jaramillo, Eric D. Bloch, Michael Huynh, David Gygi, Cyrille Costentin, and Daniel G. Nocera
Department of Chemistry and Chemical Biology, Harvard University, Cambridge, Massachusetts 02138, USA
Laboratoire d'Electrochimie Moléculaire, Unité Mixte de Recherche Université – CNRS N° 7591, Bâtiment Lavoisier, Université Paris Diderot, Sorbonne Paris Cité, 15 rue Jean de Baïf, 75205 Paris Cedex 13, France
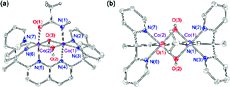
The mechanism of oxygen activation at a dicobalt bis-μ-hydroxo core is probed by the implementation of synthetic methods to isolate reaction intermediates. Reduction of a dicobalt(III,III) core ligated by the polypyridyl ligand dipyridylethane naphthyridine (DPEN) by two electrons and subsequent protonation result in the release of one water moiety to furnish a dicobalt(II,II) center with an open binding site. This reduced core may be independently isolated by chemical reduction. Variable-temperature 1H NMR and SQUID magnetometry reveal the reduced dicobalt(II,II) intermediate to consist of two low spin Co(II) centers coupled antiferromagnetically. Binding of O2 to the open coordination site of the dicobalt(II,II) core results in the production of an oxygen adduct, which is proposed to be a dicobalt(III,III) peroxo. Electrochemical studies show that the addition of two electrons results in cleavage of the O–O bond. |

