Publication
862
ChemistrySelect, 3 (31), 8874-8880, 2018
DOI:10.1002/slct.201801957
|
|
|
|
|
|
|
Electrochemical Activation of TTF-Based Halogen Bond Donors: A Powerful, Selective and Sensitive Analytical Tool for Probing a Weak Interaction in Complex Media
|
|
|
|
Raquel Oliveira, Sihem Groni, Antoine Vacher, Frédéric Barrière, Dominique Lorcy, Marc Fourmigué, Emmanuel Maisonhaute, Bernd Schöllhorn, and Claire Fave
Laboratoire d'Electrochimie Moléculaire, UMR CNRS 7591, Université Paris Diderot, Sorbonne Paris Cité, 15 rue Jean-Antoine de Baïf, France
Université Rennes, CNRS, ISCR Institut des Sciences Chimiques de Rennes - UMR 6226, France
Sorbonne Université, CNRS, Laboratoire Interfaces et Systèmes Electrochimiques, LISE, France
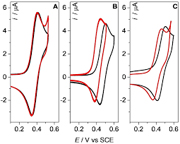
Herein we demonstrate the electrochemical activation and detection of intermolecular halogen bonding (XB) in solution by generating the cation radical of iodo-trimethyltetrathiafulvalene (I-Me3TTF•+) in the presence of various Lewis basic anions. This work is leading to the endorsement and generalization of the concept of electrostimulating XB for molecular recognition by adjusting the properties of the XB donor via the electrochemical reversible generation of charged species. The formation of the interaction between I-Me3TTF•+ and Lewis bases has been investigated by cyclic voltammetry allowing to quantify the strength of the interaction (KOx=100 to 400 M-1 and DeltaG=-12 to -15 kJ mol-1). Moreover spectroelectrochemical measurements along with TD-DFT calculations have confirmed XB formation upon generation of the radical cation. The present work opens broad perspectives for the development of redox switchable XB donors in the field of molecular recognition in chemical and biological systems where non-covalent interactions are usually key governing parameters. |

