Publication
863
J. Am. Chem. Soc. 140 (42), 13711-13718, 2018
DOI:10.1021/jacs.8b07149
|
|
|
|
|
|
|
Direct Electrochemical P(V) to P(III) Reduction of Phosphine Oxide Facilitated by Triaryl Borates
|
|
|
|
Joseph S. Elias, Cyrille Costentin, and Daniel G. Nocera
Department of Chemistry and Chemical Biology, Harvard University, Cambridge, Massachusetts 02138, United States
Laboratoire d’Electrochimie Moléculaire, Unité Mixte de Recherche Université−CNRS 7591, Université Paris Diderot, Sorbonne Paris Cité, 75205 Paris Cedex 13, France
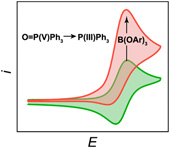
Triaryl borate Lewis acids facilitate the direct two-electron reduction of the P(V) center of triphenylphosphine oxide (TPPO) to the P(III) center of triphenylphosphine at faradaic efficiencies of 37%. Insight from direct P(V) to P(III) reduction is provided from cyclic voltammetry. The electrochemical reduction of TPPO proceeds through an unusual ECrECi mechanism in which the breaking of the phosphoryl bond in a two-electron-reduced association complex with the triaryl borate is rate-determining. The rate and faradaic efficiency for TPPO reduction are tuned by judicious choice of substituents on triaryl borate, with tris(4-methoxyphenyl) borate demonstrating the highest for both. These results suggest that an attractive route toward the room-temperature reduction of phosphate for phosphorus reclamation is greatly facilitated by the stabilization of reduced phosphate intermediates through their association with Lewis acids. |

