Publication
864
Proc. Natl. Acad. Sci. U.S.A. 115 (37), 9104-9109, 2018
DOI:10.1073/pnas.1810255115
|
|
|
|
|
|
|
Ligand “noninnocence” in coordination complexes vs. kinetic, mechanistic, and selectivity issues in electrochemical catalysis
|
|
|
|
Cyrille Costentin, Jean-Michel Savéant, and Cédric Tard
Université Paris Diderot, Sorbonne Paris Cité, Laboratoire d’Electrochimie Moléculaire, Unité Mixte de Recherche Université, CNRS 7591, 75205 Paris Cedex 13, France
LCM, CNRS, Ecole Polytechnique, Université Paris-Saclay, 91128 Palaiseau Cedex, France
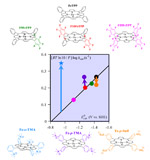
The world of coordination complexes is currently stimulated by the quest for efficient catalysts for the electrochemical reactions underlying modern energy and environmental challenges. Even in the case of a multielectron−multistep process, catalysis starts with uptake or removal of one electron from the resting state of the catalyst. If this first step is an outer-sphere electron transfer (triggering a “redox catalysis” process), the electron distribution over the metal and the ligand is of minor importance. This is no longer the case with “chemical catalysis,” in which the active catalyst reacts with the substrate in an inner-sphere manner, often involving the transient formation of a catalyst−substrate adduct. The fact that, in most cases, the ligand is “noninnocent,” in the sense that the electron density and charge gained (or removed) from the resting state of the catalyst are shared between the metal and the ligand, has become common-place knowledge over the last half-century. Insistent focus on a large degree of noninnocence of the ligand in the resting state of the catalyst, even robustly validated by spectroscopic techniques, may lead to undermining the essential role of the metal when such essential issues as kinetics, mechanisms, and product selectivity are dealt with. These points are general in scope, but their discussion is eased by adequately documented examples. This is the case for reactions involving metalloporphyrins as well as vitamin B12 derivatives and similar cobalt complexes for which a wealth of experimental data is available. |

