Publication
866
Frontiers in Chemistry, 6, 590, 2018
DOI:10.3389/fchem.2018.00590
|
|
|
|
|
|
|
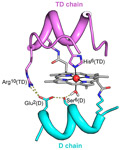
Mn-Mimochrome VI*a: An Artificial Metalloenzyme With Peroxygenase Activity
|
|
|
|
Leone Linda, D'Alonzo Daniele, Balland Véronique, Zambrano Gerardo, Chino Marco, Nastri Flavia, Maglio Ornella, Pavone Vincenzo, Lombardi Angela
Department of Chemical Sciences, University of Naples “Federico II”, Naples, Italy
Laboratoire d'Electrochimie Moléculaire, UMR 7591 CNRS, Université Paris Diderot, Sorbonne Paris Cité, Paris, France
Institute of Biostructures and Bioimages, National Research Council, Naples, Italy
Manganese-porphyrins are important tools in catalysis, due to their capability to promote a wide variety of synthetically valuable transformations. Despite their great reactivity, the difficulties to control the reaction selectivity and to protect the catalyst from self-degradation hamper their practical application. Respect to small-molecule porphyrin complexes, metalloenzymes display remarkable features because the reactivity of the metal center is finely modulated by a complex interplay of interactions within the protein matrix. In the effort to combine the catalytic potential of manganese porphyrins with the unique properties of biological catalysts, artificial metalloenzymes have been reported, mainly by incorporation of manganese-porphyrins into native protein scaffolds. Here we describe the spectroscopic and catalytic properties of Mn-Mimochrome VI*a (Mn-MC6*a), a mini-protein with a manganese deuteroporphyrin active site within a scaffold of two synthetic peptides covalently bound to the porphyrin. Mn-MC6*a is an efficient catalyst endowed with peroxygenase activity. The UV-vis absorption profile of Mn-MC6*a resembles that of Mn-reconstituted horseradish peroxidase (Mn-HRP), both in the resting and high-valent oxidized states. Remarkably, Mn-MC6*a shows a higher reactivity compared to Mn-HRP, because higher yields and chemoselectivity were observed in thioether oxidation. Experimental evidences also provided indications on the nature of the high-valent reactive intermediate and on the sulfoxidation mechanisms. |

