Publication
867
Electrochim. Acta, 299, 222-230, 2019
DOI:10.1016/j.electacta.2018.12.105
|
|
|
|
|
|
|
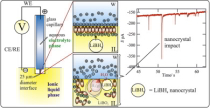
Single LiBH4 nanocrystal stochastic impacts at a micro water|ionic liquid interface
|
|
|
|
T. Jane Stockmann, Jean-François Lemineur, Huiyin Liu, Claudio Cometto, Marc Robert, Catherine Combellas, and Frédéric Kanoufi
Memorial University of Newfoundland, Department of Chemistry, St. John's NL, A1B 3X7 Canada
Sorbonne Paris Cité, Paris Diderot University, Interfaces, Traitements, Organisation et Dynamique des Systèmes, CNRS-UMR 7086 15 rue J. A. Baif, 75013 Paris, France
Sorbonne Paris Cité, Laboratoire d’Electrochimie Moléculaire, UMR 7591 Université Paris Diderot - CNRS, 15 rue J.A. de Baïf, 75013 Paris, France
LiBH4 is often employed as a reducing agent for metal nanoparticle (NP) preparation but is inherently a solid-state H2 hydrogen storage agent. Herein it is shown, through a combination of electron/optical microscopies and single entity electrochemical study, that LiBH4 is stored in the solid state within an ionic liquid (IL) as nanocrystals (NCs). The electrochemical monitoring of an immiscible water|IL (w|IL) micro-liquid|liquid interface (LLI) shows interfacial charge exchange associated with the stochastic impacts of single NCs. Meanwhile, in situ optical monitoring of a w|metal or w|IL interface shows that such impacts are associated with the development of a H2-in-IL micro/nano-foam related to the poor solubility of H2. Both the presence of solid NCs and the latter H2-in-IL foam suggest that H2 release from LiBH4-in-IL is a slow, but likely controlled process. The rate of H2 production at a macroscopic LLI is further confirmed by gas chromatographic measurements, in very good agreement with microscopic observations. The electrochemical LLI provides unique investigative access to LiBH4 NCs and offers insight into H2 storage in ILs, or for direct borohydride fuel cells, as well as NP synthesis. |

