Publication
874
Organometallics, 38 (6), 1280-1285, 2019
DOI:10.1021/acs.organomet.8b00555
|
|
|
|
|
|
|
Molecular Electrochemical Catalysis of the CO2-to-CO Conversion with a Co Complex: A Cyclic Voltammetry Mechanistic Investigation |
|
|
|
Claudio Cometto, Lingjing Chen, Elodie Anxolabéhère-Mallart, Claire Fave, Tai-Chu Lau, and Marc Robert
Sorbonne Paris Cité, Laboratoire d’Electrochimie Moléculaire, UMR 7591 CNRS, Université Paris Diderot, 15 rue Jean-Antoine de Baïf, Cedex 13 F-75205 Paris, France
School of Environment and Civil Engineering, Dongguan University of Technology, Guangdong 523808, China
Department of Chemistry, Institute of Molecular Functional Materials, City University of Hong Kong, Tat Chee Avenue, Kowloon Tong, Hong Kong, China
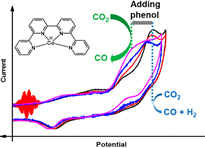
The electrochemical catalytic reduction of CO2 into CO could be achieved with excellent selectivity and rate in acetonitrile in the presence of phenol with cobalt 2,2':6',2":6",2"'-quaterpyridine complex [CoII(qpy)(H2O)2]2+ (Co) acting as a molecular catalyst. Upon using cyclic voltammetry at low and high scan rate (up to 500 V/s) two catalytic pathways have been identified. At a low concentration of phenol (<1 M), catalysis mainly occurs after the reduction of Co with three electrons. In that case, the selectivity for CO production is ca. 80% with 20% of H2 as by product, along with a turnover frequency of 1.2 x 104 s-1 for CO production at an overpotential of ca. 0.6 V. The triply reduced active species binds to CO2 and the C-O bond is cleaved thanks to the acid. At very large concentration of phenol (3 M), another pathway becomes predominant: the doubly reduced species binds to CO2, while its reductive protonation leads to CO formation. As already shown, this later process is endowed with fast rate at low overpotential (turnover frequency of 3 x 104 s-1 at an overpotential of 0.3 V) and 95% selectivity for CO production. By varying the phenol concentration and the scan rate in voltammetry experiments, it was thus possible to identify, activate, and characterize several pathways for the CO2-to-CO conversion and to decipher Co electrochemical reactivity toward CO2. |

