Publication
883
ACS Catal., 9 (6), 5783-5796, 2019
DOI:10.1021/acscatal.9b01263
|
|
|
|
|
|
|
Immuno-Based Molecular Scaffolding of Glucose Dehydrogenase and Ferrocene Mediator on fd Viral Particles Yields Enhanced Bioelectrocatalysis |
|
|
|
Kristian Torbensen, Anisha N. Patel, Agnès Anne, Arnaud Chovin, Christophe Demaille,
Laure Bataille, Thierry Michon,
and Eric Grelet
Laboratoire d'Electrochimie Moléculaire, UMR 7591 CNRS, Université Paris Diderot, Sorbonne Paris Cité, 15 rue Jean-Antoine de Baïf, F-75205 Paris Cedex 13, France
UMR 1332 Biologie du Fruit et Pathologie, INRA, Université de Bordeaux, 71, avenue Edouard Bourlaux, CS 20032-33882 Villenave d’Ornon Cedex, France
Centre de Recherche Paul-Pascal, UMR 5031 CNRS, Université de Bordeaux, 115 avenue Schweitzer, 33600 Pessac, France
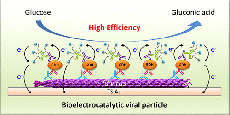
A virus-based nanostructuring strategy is proposed for improving the catalytic performance of integrated redox enzyme electrodes. Random arrays of adsorbed filamentous fd bacteriophage particles, used as scaffolds, are assembled onto gold electrode surfaces. The viral particles are endowed with functionally coupled enzymatic and redox properties, by the sequential immunological assembly of quinoprotein glucose dehydrogenase conjugated antibodies and ferrocene PEGylated antibodies on their protein shell. The resulting virus-scaffolded enzyme/redox mediator integrated system displays a large enhancement in the catalytic current generated per enzyme molecule (i.e., in enzymatic turnover) as compared with nonscaffolded integrated glucose oxidizing enzyme electrodes. The mechanism underlying the observed scaffolding-induced catalytic enhancement is deciphered. Confinement of the mediator on the viral scaffold enables fast electron transport rate and shifts the enzyme behavior into its most effective cooperative kinetic mode. |

