Publication
890
Nat. Commun., 10 (1), 3602, 2019
DOI:10.1038/s41467-019-11542-w
|
|
|
|
|
|
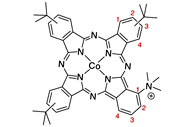 |
CO2 electrochemical catalytic reduction with a highly active cobalt phthalocyanine
|
|
|
|
Min Wang, Kristian Torbensen, Danielle Salvatore, Shaoxuan Ren, Dorian Joulié, Fabienne Dumoulin, Daniela Mendoza, Benedikt Lassalle-Kaiser, Umit Işci, Curtis P. Berlinguette, and Marc Robert
Université de Paris, Laboratoire d’Electrochimie Moléculaire, CNRS, F-75013 Paris, France
Department of Chemical and Biological Engineering, The University of British Columbia, Vancouver, BC, V6Y 1Z3, Canada
Department of Chemistry, The University of British Columbia, 2036 Main Mall, Vancouver, BC, V6T 1Z1, Canada
Gebze Technical University, Department of Chemistry, 41400, Gebze, Kocaeli, Turkey
Stewart Blusson Quantum Matter Institute, The University of British Columbia, 2355 East Mall, Vancouver, BC, V6T 1Z4, Canada
Synchrotron SOLEIL, L’Orme des Merisiers, Saint-Aubin, 91192, Gif-sur-Yvette, France
Abstract
Molecular catalysts that combine high product selectivity and high current density for CO2 electrochemical reduction to CO or other chemical feedstocks are urgently needed. While earth-abundant metal-based molecular electrocatalysts with high selectivity for CO2 to CO conversion are known, they are characterized by current densities that are significantly lower than those obtained with solid-state metal materials. Here, we report that a cobalt phthalocyanine bearing a trimethyl ammonium group appended to the phthalocyanine macrocycle is capable of reducing CO2 to CO in water with high activity over a broad pH range from 4 to 14. In a flow cell configuration operating in basic conditions, CO production occurs with excellent selectivity (ca. 95%), and good stability with a maximum partial current density of 165 mA cm-2 (at -0.92 V vs. RHE), matching the most active noble metal-based nanocatalysts. These results represent state-of-the-art performance for electrolytic carbon dioxide reduction by a molecular catalyst. |

