Publication
893
Nat. Catal., 2 (9), 801-808, 2019
DOI:10.1038/s41929-019-0331-6
|
|
|
|
|
|
|
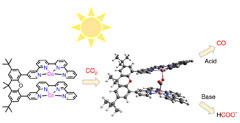
Selectivity control of CO versus HCOO- production in the visible-light-driven catalytic reduction of CO2 with two cooperative metal sites
|
|
|
|
Zhenguo Guo, Gui Chen, Claudio Cometto, Bing Ma, Hongyan Zhao, Thomas Groizard, Lingjing Chen, Hongbo Fan, Wai-Lun Man, Shek-Man Yiu, Kai-Chung Lau, Tai-Chu Lau, and Marc Robert
School of Environment and Civil Engineering, Dongguan University of Technology, Dongguan, Guangdong, China
Zhenguo Guo, Gui Chen, Lingjing Chen, Hongbo Fan & Shek-Man Yiu
Department of Chemistry and Institute of Molecular Functional Materials, City University of Hong Kong, Hong Kong, China
Université de Paris, Laboratoire d’Electrochimie Moléculaire, CNRS, Paris, France
Department of Chemistry, Hong Kong Baptist University, Kowloon Tong, Hong Kong, China
Abstract
It is highly desirable to discover molecular catalysts with controlled selectivity for visible-light-driven CO2 reduction to fuels. In the design of catalysts employing earth-abundant metals, progress has been made for CO production, but formate generation has been observed more rarely. Here, we report a binuclear Co complex bearing a bi-quaterpyridine ligand that can selectively reduce CO2 to HCOO- or CO under visible light irradiation. Selective formate production (maximum of 97%) was obtained with a turnover number of up to 821 in basic acetonitrile solution. Conversely, in the presence of a weak acid, CO2 reduction affords CO with high selectivity (maximum of 99%) and a maximum turnover number of 829. The catalytic process is controlled by the two Co atoms acting synergistically, and the selectivity can be steered towards the desired product by simply changing the acid co-substrate. |

