Publication
894
Chem. Sci., 10, 8752-8763, 2019
DOI:10.1039/C9SC02397F
|
|
|
|
|
|
|
On the unsuspected role of multivalent metal ions on the charge storage of a metal oxide electrode in mild aqueous electrolytes
|
|
|
|
Yee-Seul Kim, Kenneth D. Harris, Benoît Limoges, and Véronique Balland
Université de Paris, Laboratoire d'Electrochimie Moléculaire, UMR 7591, CNRS, F-75013 Paris, France
NRC Nanotechnology Research Centre, Edmonton, Alberta T6G 2M9, Canada
Department of Mechanical Engineering, University of Alberta, Edmonton, Alberta T6G 2V4, Canada
Abstract
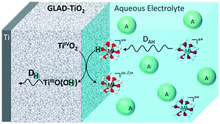
Insertion mechanisms of multivalent ions in transition metal oxide cathodes are poorly understood and subject to controversy and debate, especially when performed in aqueous electrolytes. To address this issue, we have here investigated the reversible reduction of nanostructured amorphous TiO2 electrodes by spectroelectrochemistry in mild aqueous electrolytes containing either a multivalent metal salt as AlCl3 or a weak organic acid as acetic acid. Our results show that the reversible charge storage in TiO2 is thermodynamically and kinetically indistinguishable when carried out in either an Al3+- or acetic acid-based electrolyte, both leading under similar conditions of pH and concentrations to an almost identical maximal charge storage of ~115 mA h g-1. These observations are in agreement with a mechanism where the inserting/deinserting cation is the proton and not the multivalent metal cation. Analysis of the data also demonstrates that the proton source is the Bronsted weak acid present in the aqueous electrolyte, i.e. either the acetic acid or the aquo metal ion complex generated from solvation of Al3+ (i.e. [Al(H2O)6]3+). Such a proton-coupled charge storage mechanism is also found to occur with other multivalent metal ions such as Zn2+ and Mn2+, albeit with a lower efficiency than Al3+, an effect we have attributed to the lower acidity of [Zn(H2O)6]2+ and [Mn(H2O)6]2+. These findings are of fundamental importance because they shed new light on previous studies assuming reversible Al3+-insertion into metal oxides, and, more generally, they highlight the unsuspected proton donor role played by multivalent metal cations commonly involved in rechargeable aqueous batteries.
 Themed collection: 2019 Chemical Science HOT Article Collection Themed collection: 2019 Chemical Science HOT Article Collection
 PDF Article including ChemSciCover PDF Article including ChemSciCover
|

