Publication
896
Angew. Chem. Int. Ed., 58 (45), 16172-16176, 2019
DOI:10.1002/anie.201909257
|
|
|
|
|
|
|
Aqueous Electrochemical Reduction of Carbon Dioxide and Carbon Monoxide into Methanol with Cobalt Phthalocyanine
|
|
|
|
Etienne Boutin, Min Wang, John C. Lin, Matthieu Mesnage, Daniela Mendoza, Benedikt Lassalle-Kaiser, Christopher Hahn, Thomas F. Jaramillo, and Marc Robert
Université de Paris, Laboratoire d’Electrochimie Moléculaire, CNRS 75013 Paris (France)
SUNCAT Center for Interface Science and Catalysis, Dept of Chemical Engineering, Stanford University Stanford, CA 94305 (USA)
Synchrotron SOLEIL L’Orme des Merisiers, Saint-Aubin, 91192, Gif-sur-Yvette (France)
SUNCAT Center for Interface Science and Catalysis, SLAC National Accelerator Laboratory 2575 Sand Hill Road, Menlo Park, CA 94025 (USA)
Abstract
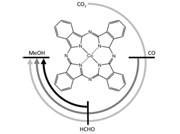
Conversion of CO2 into valuable molecules is a field of intensive investigation with the aim of developing scalable technologies for making fuels using renewable energy sources. While electrochemical reduction into CO and formate are approaching industrial maturity, a current challenge is obtaining more reduced products like methanol. However, literature on the matter is scarce, and even more for the use of molecular catalysts. Here, we demonstrate that cobalt phthalocyanine, a well-known catalyst for the electrochemical conversion of CO2 to CO, can also catalyze the reaction from CO2 or CO to methanol in aqueous electrolytes at ambient conditions of temperature and pressure. The studies identify formaldehyde as a key intermediate and an unexpected pH effect on selectivity. This paves the way for establishing a sequential process where CO2 is first converted to CO which is subsequently used as a reactant to produce methanol. Under ideal conditions, the reaction shows a global Faradaic efficiency of 19.5 % and chemical selectivity of 7.5 %. |

