Publication
899
Chem. Eur. J., 26 (14), 3034-3038, 2020
DOI:10.1002/chem.202000160
|
|
|
|
|
|
|
Iron Porphyrin Allows Fast and Selective Electrocatalytic Conversion of CO2 to CO in a Flow Cell
|
|
|
|
Kristian Torbensen, Chegn Han, Benjamin Boudy, Niklas von Wolff, Caroline. Bertail, Waldemar Braun, and Marc Robert
Laboratoire d'Electrochimie Moléculaire, Université de Paris, CNRS, 75013 Paris, France
College of Aerospace Science and Engineering, National University of Defense Technology, Changsha, Hunan, 410073 P. R. China
Air Liquide Research &Development Paris Innovation Campus, 78354 Jouy en Josas, France
Air Liquide Forschung und Entwicklung GmbH, Gwinnerstraße 27–33, 60388 Frankfurt, Germany
Abstract
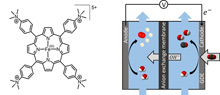
Molecular catalysts have been shown to have high selectivity for CO2 electrochemical reduction to CO, but with current densities significantly below those obtained with solid state materials. By depositing a simple Fe porphyrin mixed with carbon black onto a carbon paper support, it was possible to obtain a catalytic material that could be used in a flow cell for fast and selective conversion of CO2 to CO. At neutral pH (7.3) a current density as high as 83.7 mA cm-2 was obtained with a CO selectivity close to 98%. In basic solution (pH 14), a current density of 27 mA cm-2 was maintained for 24h with 99.7% selectivity for CO at only 50 mV overpotential, leading to a record energy efficiency of 71%. In addition, a current density for CO production as high as 152 mA cm-2 (>98% selectivity) was obtained at a low overpotential of 470 mV, outperforming state of the art noble metal based catalysts. |

