Publication
914
ChemSusChem, 13 (16 ) , 4111-4120, 2020
DOI:10.1002/cssc.202001143
|
|
|
|
|
|
|
|
Electrochemical Conversion of CO2 to CO by a Competent Fe I Intermediate Bearing a Schiff Base Ligand |
|
|
|
Ruggero Bonetto, Roberto Altieri, Mirko Tagliapietra, Antonio Barbon, Marcella Bonchio, Marc Robert, and Andrea Sartorel
Université de Paris, Laboratoire d’Electrochimie Moléculaire LEM, CNRS, 75006 Paris, France
Institut Universitaire de France (IUF), 75005 Paris, France
Department of Chemical Sciences, University of Padova, Via Marzolo, 1, 35131 Padova, Italy
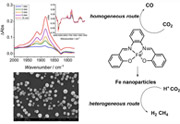
Iron complexes with a N2O2‐type N,N‐bis(salicylaldehyde)‐1,2‐phenylenediamine salophen ligand catalyze the electrochemical reduction of CO2 to CO in acetonitrile with phenol as the proton donor, giving rise to 90–99 % selectivity, faradaic efficiency up to 58 %, and turnover frequency up to 103 s−1 at an overpotential of 0.65 V. This novel class of molecular catalyst for CO2 reduction operate through a mononuclear FeI intermediate, with phenol being involved in the process with first‐order kinetics. The molecular nature of the catalyst and the low cost, easy synthesis and functionalization of the salophen ligand paves the way for catalyst engineering and optimization. Competitive electrodeposition of the coordination complex at the electrode surface results in the formation of iron‐based nanoparticles, which are active towards heterogeneous electrocatalytic processes mainly leading to proton reduction to hydrogen (faradaic efficiency up to 80 %) but also to the direct reduction of CO2 to methane with a faradaic efficiency of 1–2 %. |

