Publication
918
J. Electroanal. Chem., 872 , 114322, 2020
DOI:10.1016/j.jelechem.2020.114322
|
|
|
|
|
|
|
|
Long range self-organisations of small metallic nanocrystals for SERS detection of electrochemical reactions |
|
|
|
|
Sihem Groni, Claire Fave, Bernd Schöllhorn, Lionel Chapus, Pierre Aubertin, Thomas Touzalin, Ivan T. Lucas, Suzanne Joiret, Alexa Courty, and Emmanuel Maisonhaute
Université de Paris, Laboratoire d'Electrochimie Moléculaire, UMR CNRS 7591, 15 Rue Jean-Antoine de Baïf, F-75013 Paris, France
Sorbonne Université, CNRS, MONARIS, UMR 8233, F-75005, 4 place Jussieu, F-75005 Paris, France
Sorbonne Université, CNRS, Laboratoire Interfaces et Systèmes Electrochimiques, LISE, F-75005 Paris, France
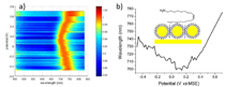
Gold electrodes were modified by silver and gold nanocrystals (NCs) that self-organize onto the surface. Their optical properties were explored by measuring electroreflectance spectra as a function of electrode potential. Below their oxidation potential, no shift of the reflectance maximum was observed for Ag NCs. This can be explained by a low interfacial capacitance resulting from the impossibility for the electrolyte to penetrate into the hydrophobic layer created by the NCs dodecanethiol ligands. Conversely, a non-monotonous evolution was observed with the electrode potential for oleylamine capped Au NCs. This behavior is suggesting a less dense hydrophobic layer, allowing significant electrolyte penetration. Next, electroactive compounds were adsorbed on the the Au NCs assemblies and characterized by Raman spectroelectrochemistry. In the first system displaying a single electron transfer with no coupled chemical reaction, only the spectrum intensity changed, because oxidation generated a Raman resonant radical cation. The second study considered 4-nitrothiophenol, for which up to 6 electrons and 6 protons may be transferred. In this case, the nitro band disappeared upon reduction, and the spectrum displayed typical features of the formed 4-aminothiophenoL. |

